Though medical advances typically outpace most fields of technology, a surprising exception has been the rudimentary technique used by obstetricians since 1958 to cut umbilical cords. This standard "old school" procedure - using multiple clamps, scissors and a few sets of hands - exposes those in the delivery room to a hazardous spray of blood in over 30% of all deliveries. With blood-borne pathogens (such as HIV, STDs and Hepatitis C) becoming an increasing risk among healthcare workers, a solution to this safety hazard has been long overdue.
Enter the Joey Spray Guard™. "This innovative medical device," explains CEO Mark Bode," is the brainchild of Dr. Richard Watson, an obstetrician who was himself sprayed in the eyes by cord blood during the delivery of a baby with an HIV-infected mother."
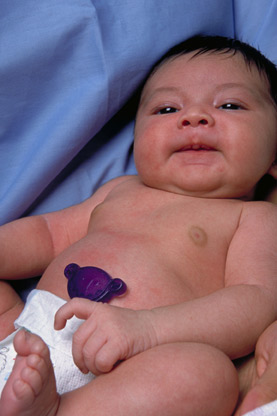
That incident inspired Watson's to design the one-piece clamping and cutting Joey Spray Guard™, a single-handed tool which nearly eliminates the exposure risk and leaves behind a koala face clamp attached to the newborn's belly.
Bode, whose background includes more than 20 years of venture capital experience, was involved in an advisory capacity with Maternus Medical Ltd, the company that first developed this breakthrough device. He, along with other members of the JoeyMedical team (including wife, Karen) advise on and acquire technologies they believe will have strong economic and social potential. They knew the Joey was something special.
Confirming their instincts were the many awards the Joey had already received. In July 2008, Bode and his team of experts followed their intuition. Obtaining the exclusive worldwide rights to produce and sell the Joey Spray Guard™, the JoeyMedical team brought their "newborn" home to Cincinnati.
Since its commercial release in May 2009, the Joey Spray Guard™ has received international attention. Trials of the device have been undertaken at such renowned medical facilities as John Hopkins and the Mayo Clinic. In June 2010, the Air Force began its own study of the Joey.
"We are excited to receive the validation of our product from such an excellent team," says Bode. In October 2010, the top obstetrics team of the Air Force will present an abstract on the Joey Spray Guard™ to a respected group of 200 military obstetricians.
Already cleared by the Food and Drug Administration (FDA) and CE approved, Joey is being distributed to clinics worldwide, including locations in South Africa, Guatemala and the Amazon. Bode is most excited about "the life-saving impact Joey will have for caregivers at high risk in these countries."
Humanitarians at heart, both Bode and his wife say that the ultimate goal of the JoeyMedical team is to "use our profits from the sale of the Joey Spray Guard™ to help provide this safety device to the millions who couldn't otherwise afford it."
Though patiently admitting, "We have a long road ahead," Bode is understandably confident in the future of the Joey Spray Guard™ and believes that "it will be the standard of birthing worldwide."
Judging by the captivating smile on the face of his product, the future of JoeyMedical's baby koala looks very bright.
Writer: Alyce Vilines
Source: Mark Bode, CEO-Joey Medical LLC